Abstract
Cancer stem cells present a major therapeutic challenge - their effective eradication depends on therapeutic targeting of the relative vulnerabilities of the cancer stem cells vs. normal tissue stem cells. One feature that distinguishes cancer initiating and propagating cells from their normal counterparts is transcriptional addiction, providing opportunities for novel therapeutic interventions with the aim of curing cancer.
CKIα ablation appears as a promising means of activating p53 and killing leukemia cells in MDS and AML (Elyada et al , doi:10.1038/nature09673; Kronke et al, doi: 10.1038/nature14610); yet, with no selective CKIα inhibitors for in vivo use, the therapeutic value of CKIα inhibition cannot be validated. We succeeded in developing two different classes of CKIα inhibitors, one inducing CKIα degradation, and another targeting the catalytic pocket of CKIα, with no degradation. Both inhibitor types activate p53, yet we found that CKIα ablation or degradation was not sufficient to selectively eliminate leukemia stem cells, due to robust expression of anti-apoptotic oncogenes driven by super-enhancer activation.
Through extensive medicinal chemistry, we obtained CKIα catalytic inhibitors with profound killing capacity of primary mouse AML cells and human AML cell lines. We demonstrated a strong therapeutic effect in a mouse model of MLL-AF9-induced leukemia, with >40% cure rates evident by transplantation of bone marrow from treated leukemic mice to lethally irradiated normal mice. All transplanted mice regained normal blood counts, exclusively derived from the donor bone marrow. None showed any evidence of residual disease for 6 months, demonstrating a successful therapeutic window, distinguishing leukemia stem cells (LSCs) from HSPCs. Parallel in vitro experiments, show that the inhibitors did not affect normal hematopoietic progenitors in a CFU assay, but eliminated AML progenitors at an IC50 <9nM.
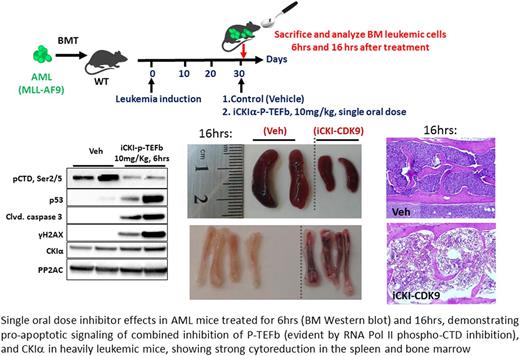
We found that CKIα inhibitors possessing potent anti-leukemia activity are distinguished by their capacity to co-target CDK9 and suppress the RNA Pol II elongation factor P-TEFb (CDK9-CyclinT1 complex), evident by blocking the phosphorylation of RNA Pol II C-terminal domain (pCTD2/5, see Figure). Co-crystallography studies elucidated the structural basis for co-targeting of CKIα and P-TEFb, the latter controls the rate limiting step of Poll-II-transcription and functions as a gatekeeper of super-enhancers. We found that both P-TEFb and super-enhancer activity are vigorously activated in AML cells. Therefore, the efficient P-TEFb-targeting activity of the inhibitors enables a selective blockade of leukemia super-enhancers (SE)-driven transcription, demonstrated by ChIP-seq analysis. As a result, SE-dependent transcription of major leukemia drivers including Myc and the anti-apoptotic oncogenes Bcl-2 and Mcl-1 was nearly abolished.
Another remarkable feature of these dual CKIα-P-TEFb inhibitors is a rapid hit mechanism, by which, short drug exposure (10 mins in vitro and 2hrs in vivo) results in prolonged (24hrs) SE suppression and leukemia cell apoptosis. This unique property, which is at variance with the current occupancy-driven pharmacological paradigm, likely contributes to the dramatic therapeutic effect of co-targeting CKIα and P-TEFb in leukemia, whereby combination of rapid p53 induction with disruption of oncogenic super-enhancers eliminates leukemia stem cells without compromising normal HSPCs. The ability to disrupt super-enhancers and eliminate their associated oncogenes within minutes of drug exposure permits short intermittent therapy, which minimizes generalized toxicity. Indeed, at the therapeutic dose, we did not observe any significant adverse treatment effects even at daily treatment for 45 days in mice.
To conclude, we developed a new class of small molecule inhibitors that co-targets CKIα and P-TEFb. These inhibitors have unique pharmacologic properties: short-term kinase inhibition results in long-term disruption of SE activity. Shutdown of leukemic super-enhancers in synergy with robust p53 activation compromises leukemic cells and stem cells addicted to SE-driven transcription. These features explain the powerful and specific anti-leukemic therapeutic effects of this new class of inhibitors in-vivo.
Ben-Neriah: BioTheryX Inc.: Consultancy, Equity Ownership, Research Funding. Vacca: BioTheryX Inc.: Consultancy. Mercurio: BioTheryX: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal